If you’ve ever had to chase down a missing courier package, you know how frustrating it is to wonder: Where exactly is it? Who signed for it? Is it lost forever?
Now, imagine that confusion not with a T-shirt order, but with Active Pharmaceutical Ingredients (APIs) the raw materials behind medicines that millions rely on. That’s exactly why India has rolled out a QR code mandate for APIs. It’s about accountability, transparency, and most importantly, trust.
From 2023 onwards, every pack of APIs whether it’s a drum, a carton, or a pallet must carry a machine-readable QR code. These aren’t just barcodes; they’re like passports that hold crucial details about what’s inside, where it came from, and where it’s headed.
Let’s unpack what this means for the industry, why blockchain “sterilization” is being talked about as the next leap, and how companies like Arvo are helping pharma players navigate the complexity with end-to-end traceability, anti-counterfeiting, and product authentication solutions.
Why India is Betting Big on QR Codes
Counterfeit medicines aren’t a small problem. The World Health Organization estimates that 1 in 10 medical products in low- and middle-income countries is substandard or falsified. In India home to one of the world’s largest pharma manufacturing ecosystems this isn’t just a local issue, it’s global.
The government’s answer? QR codes on APIs.
Here’s what each code carries:
- API name and brand name (if applicable)
- Manufacturer’s name and address
- Batch number and size
- Manufacturing date and expiry/retest date
- Manufacturing or import license number
- Special storage instructions
- Serial Shipping Container Code (SSCC)
In other words, scan that square and you’ve got an instant history lesson about the product you’re holding.
Making Compliance Practical: Explaining It Simply
1. Think GS1, not guesswork
APIs need unique identifiers, the equivalent of Aadhaar numbers for products. GS1 provides these IDs (GTINs, GLNs, SSCCs) that make your packs globally recognizable.
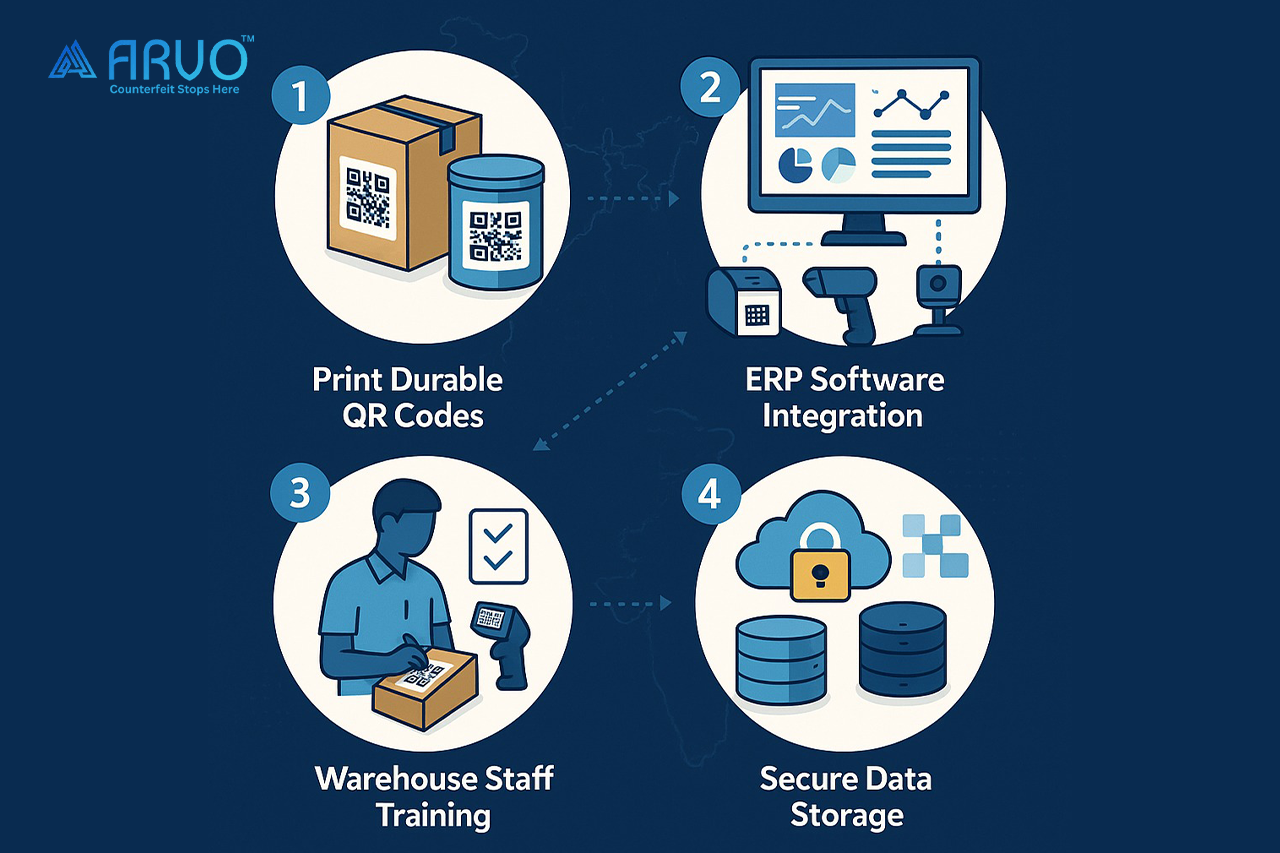
2. Print codes that survive reality
It’s one thing to print a QR code. It’s another to make sure it doesn’t smear when exposed to solvents or fade in a humid warehouse. That’s where material testing and print validation come in.
3. Keep all your data in one “source of truth”
Imagine if every department kept their own Excel sheet. Chaos, right? You need validated serialization software where all data lives securely and can’t be casually edited.
4. Connect your hardware and ERP
Printers, scanners, and vision systems should talk to your ERP/WMS in real time. That way, when a carton is printed or scanned, the data instantly updates.
5. Train your people, it’s not just about machines
A fancy QR system fails if warehouse staff peel and reapply labels incorrectly. SOPs and training sessions are your unsung heroes here.
6. Be future-ready
Regulators are moving towards digital inspection systems where they scan your packs and instantly see compliance. The data needs to be clean and accessible.
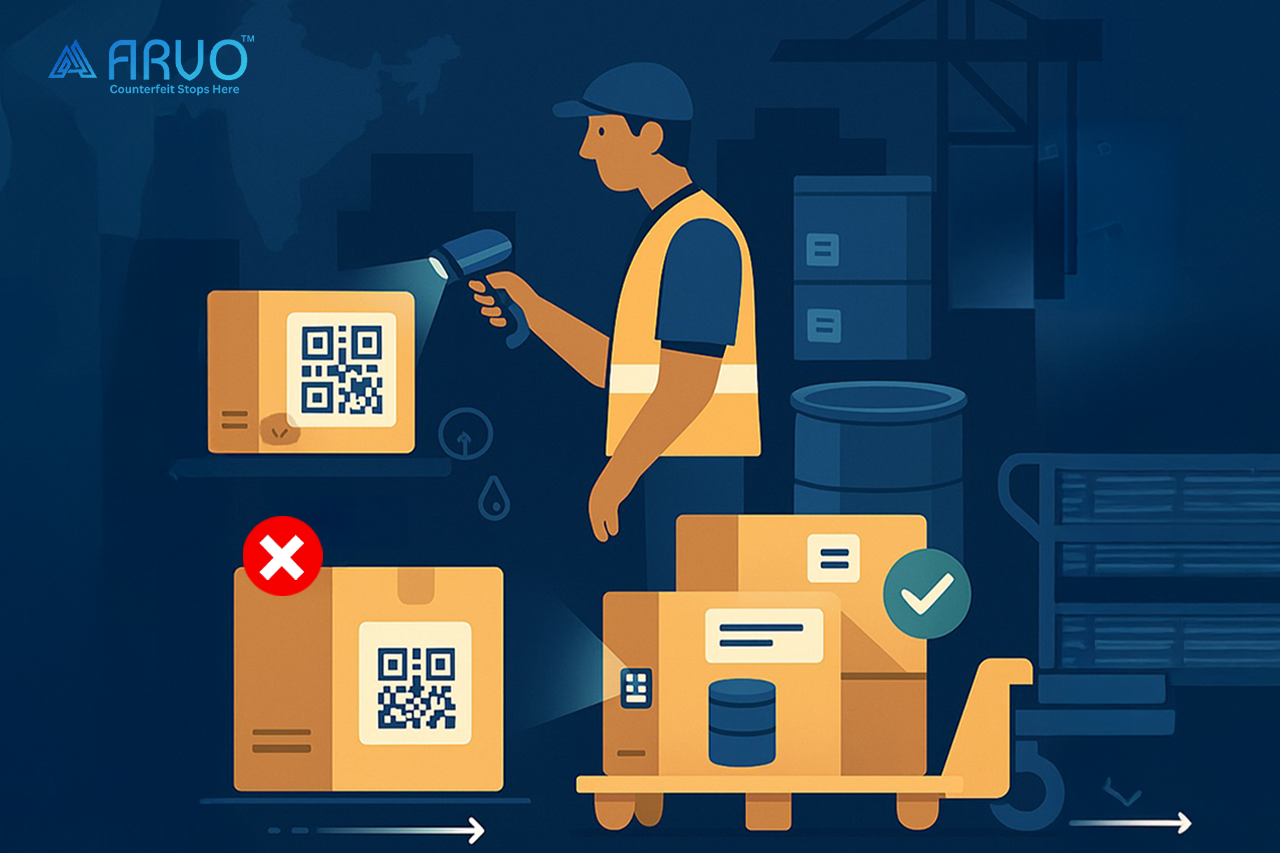
The Human Angle: What This Looks Like on the Ground
A compliance officer at a mid-sized API manufacturer recently told me about a batch that was held up at a port because the codes on a few cartons weren’t scannable. The shipment sat in customs for two weeks. Two weeks of storage costs. Two weeks of missed deliveries. All because the label material wasn’t tested against the solvents used in their plant.
Another example: a multinational pharma company implemented parent-child aggregation in their QR system. That meant if a pallet’s master code was scanned, the system automatically recognized all the drums inside it. When they piloted this with a European buyer, unloading time dropped by nearly 40%. The buyer was so impressed they made it mandatory for all suppliers.
These are small, real-world cases that show compliance isn’t just about ticking boxes. Done right, it saves money, builds trust, and even wins new business.
The Catch: QR Codes Aren’t Bulletproof
Here’s the uncomfortable truth: while QR codes add visibility, they’re not invincible. Codes can still be copied. Databases can still be tampered with.
This is where I like to bring in the idea of blockchain sterilization.
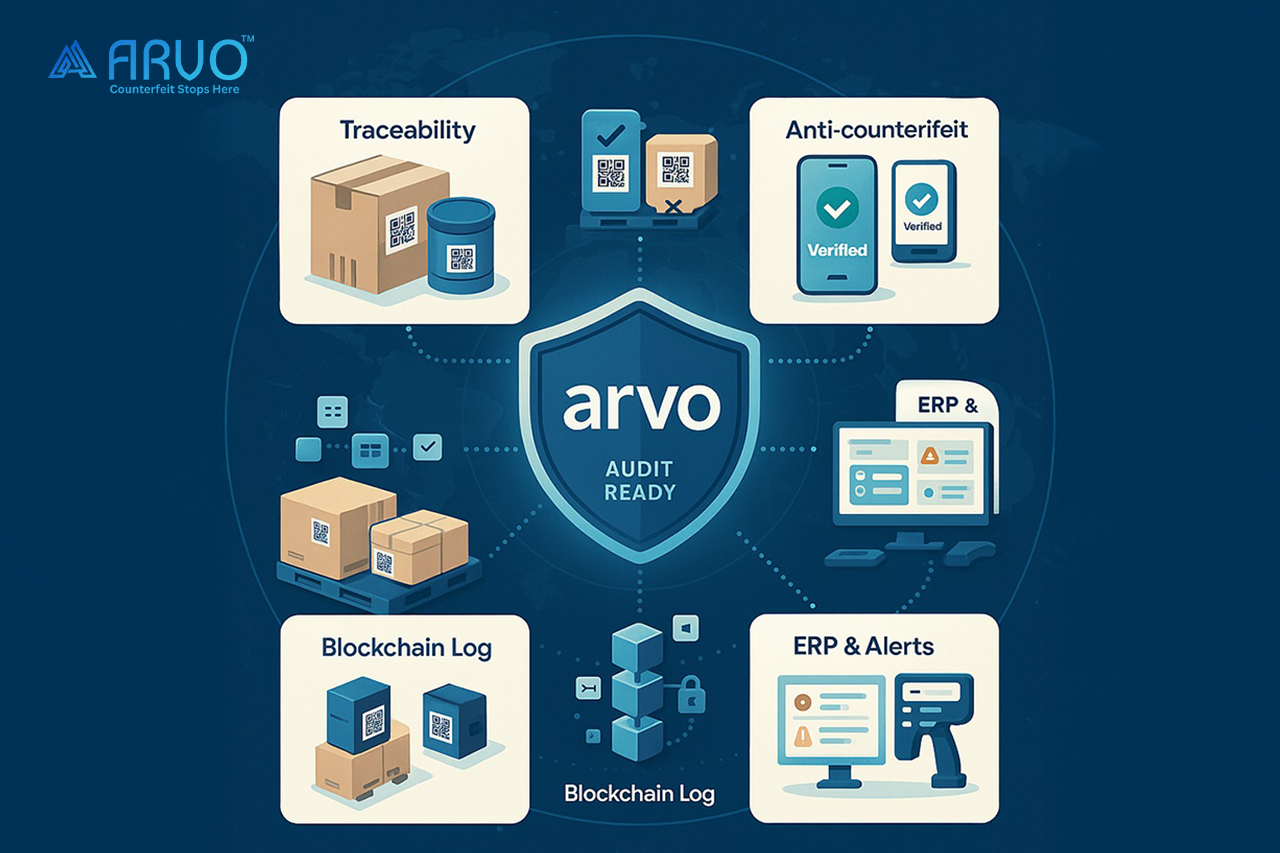
Blockchain Sterilization: Disinfecting the Data Trail
“Sterilization” usually makes us think of killing germs. In traceability, it means something different: making your digital records tamper-evident.
Here’s how it works:
- Every event printing a label, packing a carton, shipping a pallet creates a digital record.
- That record is given a cryptographic hash (like a digital fingerprint).
- The hash is stored in a blockchain ledger that no one can alter without leaving evidence.
- Your actual data sits in your ERP or serialization system, but auditors and partners can always check the blockchain to confirm it hasn’t been fiddled with.
Think of it as putting CCTV cameras on your supply chain’s digital backbone.
Note: Standard QR codes aren’t foolproof. We offer an industry-first printable non-clonable QR code.
Why Pair QR with Blockchain?
Because together, they give you the best of both worlds:
- QR code: The physical marker anyone can scan.
- Blockchain sterilization: The invisible guard ensures the scanned data is authentic.
This pairing strengthens compliance, reduces counterfeiting risks, and makes you audit-ready in a way that regulators (and overseas buyers) love.
Where This Becomes a Competitive Edge
One pharma exporter I spoke with said buyers in Europe started preferring suppliers who could provide both QR traceability and blockchain-backed data. Why? Because it reduced the buyer’s own compliance headaches. That supplier didn’t just follow the rule; they turned compliance into a selling point.
Similarly, a domestic distributor shared that when hospital procurement teams realized they could verify products instantly with QR scans tied to blockchain records, their confidence in that distributor shot up. They went from being “one of many” suppliers to the preferred partner.

Wrapping It Up: Compliance, Trust, and ARVO
The QR code mandate for APIs in India is more than just another rule. It’s a chance for pharma companies to build a transparent, trustworthy supply chain that not only satisfies regulators but also wins customer loyalty.
But here’s the fact, compliance is not always straightforward. Labels fail. Databases get messy. Records go missing. And that’s exactly where end-to-end traceability solutions come in.
This is what ARVO specializes in. Whether it’s:
- An end-to-end traceability solution that connects your packaging lines to your ERP and beyond
- An anti-counterfeiting solution that locks down your supply chain from fake product infiltration
- A product authentication solution that lets anyone regulator, distributor, or consumer verify authenticity in real time
ARVO provides the tools to not just meet the QR mandate but to go beyond it. Because at the end of the day, it’s not about printing squares on cartons. It’s about building a system of trust, accountability, and readiness for audits, for buyers, and most importantly, for the patients whose health depends on these medicines being real, safe, and traceable.
FREQUENTLY ASKED QUESTIONS (FAQ)
Q1. What is the API QR code mandate in India?
A: From 2023, the Government of India requires every pack of Active Pharmaceutical Ingredients (APIs) from drums to pallets to carry a machine-readable QR code. The code stores details like the API name, batch number, manufacturer, expiry date, and Serial Shipping Container Code (SSCC), ensuring that the product can be tracked and traced across the entire supply chain.
Q2. Why did India introduce QR codes for APIs?
A: The main goal is to fight counterfeit and substandard medicines. Fake APIs entering the pharma supply chain can compromise patient safety and damage brand trust. QR codes give regulators, manufacturers, and buyers the ability to instantly verify product authenticity and origin.
Q3. What is “blockchain sterilization” in traceability?
A: Blockchain sterilization means making your supply chain data tamper-evident. Every action like printing a label or shipping a pallet creates a digital record. That record’s fingerprint (hash) is stored on the blockchain, so if anyone tries to alter the data later, it becomes instantly visible. Think of it as putting a digital CCTV camera on your data trail.
Q4. How do QR codes and blockchain work together?
A: QR codes give each pack a visible, scannable identity. Blockchain ensures that the data behind those codes hasn’t been tampered with. Together, they create a trustworthy,
audit-ready trail that protects against counterfeiting and makes compliance seamless.
Q5. How does Arvo help companies comply with these regulations?
A: Arvo provides end-to-end traceability solutions that connect your packaging line to your ERP, anti-counterfeiting solutions that secure your supply chain, and product authentication solutions that allow anyone, regulator, distributor, or consumer to verify authenticity in real time. It’s not just compliance; it’s building trust across the pharma ecosystem.